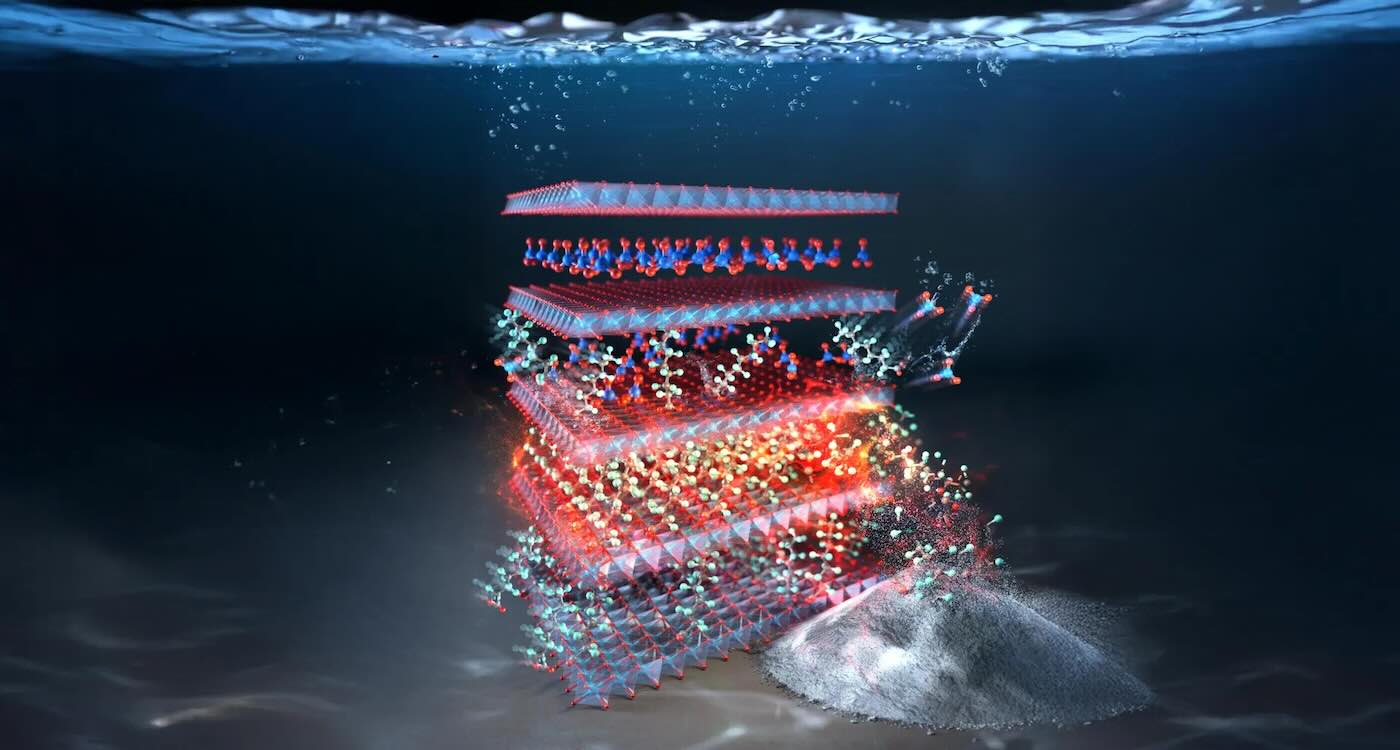
 PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice University
PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice UniversityNew Eco-Friendly Tech Eliminates ‘Forever Chemicals’ With Record-Breaking Speed–And it’s Reusable
 PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice University
PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice UniversityTime of day may determine heart surgery outcomes: Study

A Rare Cancer-Fighting Plant Compound has Finally Been Decoded
 Anti-cancer plant enzyme uncovered by Tuan-Anh Nguyen and Dr Thu-Thuy Dang – UBC Okanagan
Anti-cancer plant enzyme uncovered by Tuan-Anh Nguyen and Dr Thu-Thuy Dang – UBC Okanagan Red vein kratom leaves by Jade at Thehealingeast – CC BY-SA 4.0
Red vein kratom leaves by Jade at Thehealingeast – CC BY-SA 4.0First projects selected for INL reactor experiments
_15595.jpg) (Image: INL)
(Image: INL)Nanotechnology breakthrough may boost treatment for aggressive breast cancer: Study

Simply Shining Light on Skin Can Replace Finger Pricks for People With Diabetes
 Blood-glucose monitor uses light to spare diabetes patients from finger pricks – Credit: Christine Daniloff / MIT
Blood-glucose monitor uses light to spare diabetes patients from finger pricks – Credit: Christine Daniloff / MITA Rare Cancer-Fighting Plant Compound has Finally Been Decoded


Nearly 3x More Encounters With Endangered Sumatran Tigers in Camera Trap Photos Than in Past Years


Nagaland: Nagami becomes world’s 'first registered' Mithun breed

Polar bears are adapting to climate change at a genetic level – and it could help them avoid extinction
But in our new study my colleagues and I found that the changing climate was driving changes in the polar bear genome, potentially allowing them to more readily adapt to warmer habitats. Provided these polar bears can source enough food and breeding partners, this suggests they may potentially survive these new challenging climates.
We discovered a strong link between rising temperatures in south-east Greenland and changes in polar bear DNA. DNA is the instruction book inside every cell, guiding how an organism grows and develops. In processes called transcription and translation, DNA is copied to generate RNA (molecules that reflect gene activity) and can lead to the production of proteins, and copies of transposons (TEs), also known as “jumping genes”, which are mobile pieces of the genome that can move around and influence how other genes work.
In carrying out our recent research we found that there were big differences in the temperatures observed in the north-east, compared with the south-east regions of Greenland. Our team used publicly available polar bear genetic data from a research group at the University of Washington, US, to support our study. This dataset was generated from blood samples collected from polar bears in both northern and south-eastern Greenland.
Our work built on the Washington University study which discovered that this south-eastern population of Greenland polar bears was genetically different to the north-eastern population. South-east bears had migrated from the north and became isolated and separate approximately 200 years ago, it found.
Researchers from Washington had extracted RNA from polar bear blood samples and sequenced it. We used this RNA sequencing to look at RNA expression — the molecules that act like messengers, showing which genes are active, in relation to the climate. This gave us a detailed picture of gene activity, including the behaviour of TEs. Temperatures in Greenland have been closely monitored and recorded by the Danish Meteorological Institute. So we linked this climate data with the RNA data to explore how environmental changes may be influencing polar bear biology.
Does temperature change anything?
From our analysis we found that temperatures in the north-east of Greenland were colder and less variable, while south-east temperatures fluctuated and were significantly warmer. The figure below shows our data as well as how temperature varies across Greenland, with warmer and more volatile conditions in the south-east. This creates many challenges and changes to the habitats for the polar bears living in these regions.
In the south-east of Greenland, the ice-sheet margin, which is the edge of the ice sheet and spans 80% of Greenland, is rapidly receding, causing vast ice and habitat loss.
The loss of ice is a substantial problem for the polar bears, as this reduces the availability of hunting platforms to catch seals, leading to isolation and food scarcity. The north-east of Greenland is a vast, flat Arctic tundra, while south-east Greenland is covered by forest tundra (the transitional zone between coniferous forest and Arctic tundra). The south-east climate has high levels of rain, wind, and steep coastal mountains.
Temperature across Greenland and bear locations
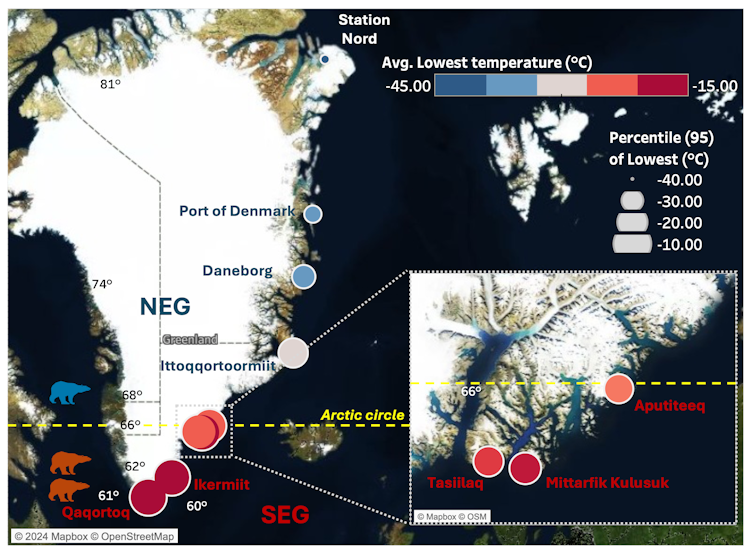 Author data visualisation using temperature data from the Danish Meteorological Institute. Locations of bears in south-east (red icons) and north-east (blue icons). CC BY-NC-ND
Author data visualisation using temperature data from the Danish Meteorological Institute. Locations of bears in south-east (red icons) and north-east (blue icons). CC BY-NC-NDHow climate is changing polar bear DNA
Over time the DNA sequence can slowly change and evolve, but environmental stress, such as warmer climate, can accelerate this process.
TEs are like puzzle pieces that can rearrange themselves, sometimes helping animals adapt to new environments. In the polar bear genome approximately 38.1% of the genome is made up of TEs. TEs come in many different families and have slightly different behaviours, but in essence they all are mobile fragments that can reinsert randomly anywhere in the genome.
In the human genome, 45% is comprised of TEs and in plants it can be over 70%. There are small protective molecules called piwi-interacting RNAs (piRNAs) that can silence the activity of TEs.
Despite this, when an environmental stress is too strong, these protective piRNAs cannot keep up with the invasive actions of TEs. In our work we found that the warmer south-east climate led to a mass mobilisation from these TEs across the polar bear genome, changing its sequence. We also found that these TE sequences appeared younger and more abundant in the south-east bears, with over 1,500 of them “upregulated”, which suggests recent genetic changes that may help bears adapt to rising temperatures.
Some of these elements overlap with genes linked to stress responses and metabolism, hinting at a possible role in coping with climate change. By studying these jumping genes, we uncovered how the polar bear genome adapts and responds, in the shorter term, to environmental stress and warmer climates.
Our research found that some genes linked to heat-stress, ageing and metabolism are behaving differently in the south-east population of polar bears. This suggests they might be adjusting to their warmer conditions. Additionally, we found active jumping genes in parts of the genome that are involved in areas tied to fat processing – important when food is scarce. This could mean that polar bears in the south-east are slowly adapting to eating the rougher plant-based diets that can be found in the warmer regions. Northern populations of bears eat mainly fatty seals.
Overall, climate change is reshaping polar bear habitats, leading to genetic changes, with south-eastern bears evolving to survive these new terrains and diets. Future research could include other polar bear populations living in challenging climates. Understanding these genetic changes help researchers see how polar bears might survive in a warming world – and which populations are most at risk.
Don’t have time to read about climate change as much as you’d like?
Get a weekly roundup in your inbox instead. Every Wednesday, The Conversation’s environment editor writes Imagine, a short email that goes a little deeper into just one climate issue. Join the 47,000+ readers who’ve subscribed so far.![]()
Alice Godden, Senior Research Associate, School of Biological Sciences, University of East Anglia
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Genetic Mutation Could Pave the Way for Self-Fertilizing Cereal Crops and a Revolution in Agriculture

Danish researchers have found a molecular switch that lets plants partner with nitrogen-fixing bacteria instead of fighting them, opening the way to self-fertilizing cereal crops like wheat and barley.
Their new research highlights an important biological clue that could help reduce agriculture’s heavy reliance on artificial nitrogen fertilizer.
Plants require nitrogen to grow, and most crop species can obtain it only through fertilizer. A small group of plants, including peas, clover, and beans, can grow without added nitrogen. They do this by forming a partnership with specific bacteria that turn nitrogen from the air into a form the plant can absorb.
In the industry, they’re known as nitrogen fixers, and crop-rotation methods dating as far back as the 17th century saw clover used to cover fields following harvests to replenish the nitrogen content of the soil.
Scientists worldwide are working to understand the genetic and molecular basis of this natural nitrogen-fixing ability. The hope is that this trait could eventually be introduced into major crops such as wheat, barley, and maize.
If achieved, these crops could supply their own nitrogen. This shift would reduce the need for synthetic fertilizer, which currently represents about 2% of global energy consumption and produces significant CO2 emissions.
That’s where the researchers at Aarhus University come in—who have now identified small receptor changes in plants that cause them to temporarily shut down their immune defenses and enter a cooperative relationship with nitrogen-fixing bacteria.
“We are one step closer to a greener and climate-friendlier food production,” said Kasper Røjkjær Andersen and Simona Radutoiu, professors of molecular biology at Aarhus University and part of the team behind the discovery.
Plants rely on cell-surface receptors to sense chemical signals from microorganisms in the soil. Some bacteria release compounds that warn the plant they are “enemies,” prompting defensive action. Others signal that they are “friends” able to supply nutrients.
Legumes such as peas, beans, and clover allow specialized bacteria to enter their roots. Inside these root tissues, the bacteria convert nitrogen from the atmosphere and share it with the plant. This partnership, known as symbiosis, is the reason legumes can grow without artificial fertilizer.
Aarhus University researchers found that this ability is strongly influenced by just two amino acids within the root protein.
“This is a remarkable and important finding,” says Radutoiu.
The root protein functions as a “receptor” that reads signals from bacteria. It determines whether the plant should activate its immune system (alarm) or accept the bacteria (symbiosis).
The team identified a small region in the receptor protein that they named Symbiosis Determinant 1. This region functions like a switch that controls which internal message the plant receives.
By modifying only two amino acids within this switch, the researchers changed a receptor that normally triggers immunity so that it instead initiated symbiosis with nitrogen-fixing bacteria in a way the plant’s natural behavior would never permit.
“We have shown that two small changes can cause plants to alter their behavior on a crucial point from rejecting bacteria to cooperating with them,” Radutoiu explains.
In laboratory experiments, the researchers successfully engineered this change in the plant Lotus japonicus. They then tested the concept in barley and found that the mechanism worked there as well.
“It is quite remarkable that we are now able to take a receptor from barley, make small changes in it, and then nitrogen fixation works again,” says Kasper Røjkjær Andersen.
The long-term potential is significant. If these modifications can be applied to other cereals, it may ultimately be possible to breed wheat, maize, or rice capable of fixing nitrogen on their own, similar to legumes.“But we have to find the other, essential keys first,” Radutoiu notes. “Only very few crops can perform symbiosis today. If we can extend that to widely used crops, it can really make a big difference on how much nitrogen needs to be used.” Genetic Mutation Could Pave the Way for Self-Fertilizing Cereal Crops and a Revolution in Agriculture
The science of weight loss – and why your brain is wired to keep you fat

For decades, we’ve been told that weight loss is a matter of willpower: eat less, move more. But modern science has proven this isn’t actually the case.
More on that in a moment. But first, let’s go back a few hundred thousand years to examine our early human ancestors. Because we can blame a lot of the difficulty we have with weight loss today on our predecessors of the past – maybe the ultimate case of blame the parents.
For our early ancestors, body fat was a lifeline: too little could mean starvation, too much could slow you down. Over time, the human body became remarkably good at guarding its energy reserves through complex biological defences wired into the brain. But in a world where food is everywhere and movement is optional, those same systems that once helped us survive uncertainty now make it difficult to lose weight.
When someone loses weight, the body reacts as if it were a threat to survival. Hunger hormones surge, food cravings intensify and energy expenditure drops. These adaptations evolved to optimise energy storage and usage in environments with fluctuating food availability. But today, with our easy access to cheap, calorie-dense junk food and sedentary routines, those same adaptations that once helped us to survive can cause us a few issues.
As we found in our recent research, our brains also have powerful mechanisms for defending body weight – and can sort of “remember” what that weight used to be. For our ancient ancestors, this meant that if weight was lost in hard times, their bodies would be able to “get back” to their usual weight during better times.
But for us modern humans, it means that our brains and bodies remember any excess weight gain as though our survival and lives depend upon it. So in effect, once the body has been heavier, the brain comes to treat that higher weight as the new normal – a level it feels compelled to defend.
The fact that our bodies have this capacity to “remember” our previous heavier weight helps to explain why so many people regain weight after dieting. But as the science shows, this weight regain is not due to a lack of discipline; rather, our biology is doing exactly what it evolved to do: defend against weight loss.
Hacking biology
This is where weight-loss medications such as Wegovy and Mounjaro have offered fresh hope. They work by mimicking gut hormones that tell the brain to curb appetite.
But not everyone responds well to such drugs. For some, the side effects can make them difficult to stick with, and for others, the drugs don’t seem to lead to weight loss at all. It’s also often the case that once treatment stops, biology reasserts itself – and the lost weight returns.
Advances in obesity and metabolism research may mean that it’s possible for future therapies to be able to turn down these signals that drive the body back to its original weight, even beyond the treatment period.
Research is also showing that good health isn’t the same thing as “a good weight”. As in, exercise, good sleep, balanced nutrition, and mental wellbeing can all improve heart and metabolic health, even if the number on the scales barely moves.
A whole society approach
Of course, obesity isn’t just an individual problem – it takes a society-wide approach to truly tackle the root causes. And research suggests that a number of preventative measures might make a difference – things such as investing in healthier school meals, reducing the marketing of junk food to children, designing neighbourhoods where walking and cycling are prioritised over cars, and restaurants having standardised food portions.
Scientists are also paying close attention to key early-life stages – from pregnancy to around the age of seven – when a child’s weight regulation system is particularly malleable.
Indeed, research has found that things like what parents eat, how infants are fed, and early lifestyle habits can all shape how the brain controls appetite and fat storage for years to come.
If you’re looking to lose weight, there are still things you can do – mainly by focusing less on crash diets and more on sustainable habits that support overall wellbeing. Prioritising sleep helps regulate appetite, for example, while regular activity – even walking – can improve your blood sugar levels and heart health.
The bottom line though is that obesity is not a personal failure, but rather a biological condition shaped by our brains, our genes, and the environments we live in. The good news is that advances in neuroscience and pharmacology are offering new opportunities in terms of treatments, while prevention strategies can shift the landscape for future generations.
So if you’ve struggled to lose weight and keep it off, know that you’re not alone, and it’s not your fault. The brain is a formidable opponent. But with science, medicine and smarter policies, we’re beginning to change the rules of the game.
This article was commissioned as part of a partnership collaboration between Videnskab.dk and The Conversation. You can read the Danish version of this article, here.![]()
Valdemar Brimnes Ingemann Johansen, PhD Fellow in the Faculty of Health and Medical Sciences, University of Copenhagen and Christoffer Clemmensen, Associate Professor and Group Leader, Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Indian study finds 1st evidence on how nanoplastics from single-use PET bottles harm body

COVID-19 mRNA vaccines could unlock the next revolution in cancer treatment – new research
The COVID-19 mRNA-based vaccines that saved 2.5 million lives globally during the pandemic could help spark the immune system to fight cancer. This is the surprising takeaway of a new study that we and our colleagues published in the journal Nature.
While developing mRNA vaccines for patients with brain tumors in 2016, our team, led by pediatric oncologist Elias Sayour, discovered that mRNA can train immune systems to kill tumors – even if the mRNA is not related to cancer.
Based on this finding, we hypothesized that mRNA vaccines designed to target the SARS-CoV-2 virus that causes COVID-19 might also have antitumor effects.
So we looked at clinical outcomes for more than 1,000 late-stage melanoma and lung cancer patients treated with a type of immunotherapy called immune checkpoint inhibitors. This treatment is a common approach doctors use to train the immune system to kill cancer. It does this by blocking a protein that tumor cells make to turn off immune cells, enabling the immune system to continue killing cancer.
Remarkably, patients who received either the Pfizer or Moderna mRNA-based COVID-19 vaccine within 100 days of starting immunotherapy were more than twice as likely to be alive after three years compared with those who didn’t receive either vaccine. Surprisingly, patients with tumors that don’t typically respond well to immunotherapy also saw very strong benefits, with nearly fivefold improvement in three-year overall survival. This link between improved survival and receiving a COVID-19 mRNA vaccine remained strong even after we controlled for factors like disease severity and co-occurring conditions.
To understand the underlying mechanism, we turned to animal models. We found that COVID-19 mRNA vaccines act like an alarm, triggering the body’s immune system to recognize and kill tumor cells and overcome the cancer’s ability to turn off immune cells. When combined, vaccines and immune checkpoint inhibitors coordinate to unleash the full power of the immune system to kill cancer cells.
Why it matters
Immunotherapy with immune checkpoint inhibitors has revolutionized cancer treatment over the past decade by producing cures in many patients who were previously considered incurable. However, these therapies are ineffective in patients with “cold” tumors that successfully evade immune detection.
Our findings suggest that mRNA vaccines may provide just the spark the immune system needs to turn these “cold” tumors “hot.” If validated in our upcoming clinical trial, our hope is that this widely available, low-cost intervention could extend the benefits of immunotherapy to millions of patients who otherwise would not benefit from this therapy.
Unlike vaccines for infectious diseases, which are used to prevent an infection, therapeutic cancer vaccines are used to help train the immune systems of cancer patients to better fight tumors.
We and many others are currently working hard to make personalized mRNA vaccines for patients with cancer. This involves taking a small sample of a patient’s tumor and using machine learning algorithms to predict which proteins in the tumor would be the best targets for a vaccine. However, this approach can be costly and difficult to manufacture.
In contrast, COVID-19 mRNA vaccines do not need to be personalized, are already widely available at low or no cost around the globe, and could be administered at any time during a patient’s treatment. Our findings that COVID-19 mRNA vaccines have substantial antitumor effects bring hope that they could help extend the anti-cancer benefits of mRNA vaccines to all.
What’s next
In pursuit of this goal, we are preparing to test this treatment strategy in patients with a nationwide clinical trial in people with lung cancer. People receiving an immune checkpoint inhibitor will be randomized to either receive a COVID-19 mRNA vaccine during treatment or not.
This study will tell us whether COVID-19 mRNA vaccines should be included as part of the standard of care for patients receiving an immune checkpoint inhibitor. Ultimately, we hope that this approach will help many patients who are treated with immune therapy, and especially those who currently lack effective treatment options.
This work exemplifies how a tool born from a global pandemic may provide a new weapon against cancer and rapidly extend the benefits of existing treatments to millions of patients. By harnessing a familiar vaccine in a new way, we hope to extend the lifesaving benefits of immunotherapy to cancer patients who were previously left behind.
The Research Brief is a short take on interesting academic work.![]()
Adam Grippin, Physician Scientist in Cancer Immunotherapy, The University of Texas MD Anderson Cancer Center and Christiano Marconi, Ph.D. Candidate in Immunotherapy, University of Florida
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Scorpion Venom May Provide the Next Breast Cancer Breakthrough
 – credit Marino Linic
– credit Marino LinicScientists in Brazil are currently testing to see if the venom of an Amazonian scorpion could be used to poison breast cancer tumors.
Researchers at the University of São Paulo’s Preto School of Pharmaceutical Sciences (FCFRP-USP) have long worked to clone and express proteins from rattlesnake and scorpion venom with hopes of transforming these powerful compounds into medicines.
Recently, their work identified that venom of the scorpion Brotheas amazonicus appears to attack breast cancer cells in a way similar to a widely used chemotherapy medication.
These early findings were generated through a collaboration with scientists from the National Institute for Amazonian Research (INPA) and the Amazonas State University (UEA).
“Through bioprospecting, we were able to identify a molecule in the species of this Amazonian scorpion that is similar to that found in the venoms of other scorpions and that acts against breast cancer cells,” said Eliane Candiani Arantes, a professor at FCFRP-USP and the coordinator of the project.
Arantes and her team identified two neurotoxins in scorpion venom with immunosuppressive effects. Working with collaborators at INPA and UEA, they found a peptide named BamazScplp1 in the venom of Brotheas amazonicus that appears to have anti-tumor potential.
Laboratory tests showed that the peptide’s impact on breast cancer cells was comparable to paclitaxel, a commonly prescribed chemotherapy treatment. It primarily triggers necrosis, a form of cell death previously associated with molecules from other scorpion species.
Arantes and her team have isolated other components of venoms from scorpions and from snakes that have been used to help develop other clinical applications, including an internal wound sealant that mimics the body’s natural clotting and scaffolding processes. It’s undergoing trials for use in nerve repair, bone healing, and restoring movement following spinal cord injury.Next time you see a scorpion, and think it a nasty creepy crawly that will send you to the hospital, show a bit of grace; they might help save a woman’s life some day. Scorpion Venom May Provide the Next Breast Cancer Breakthrough
Indian scientists find genetic clues to tackle oral cancer among women

Australia leads first human trial of one-time gene editing therapy to halve bad cholesterol

ICRISAT develops portable technology for testing crops' nutrition level

Parkinson's disease causes progressive changes in brain's blood vessels: Study

