
IIT Bombay’s new smart platform to help researchers decode brain diseases

New Eco-Friendly Tech Eliminates ‘Forever Chemicals’ With Record-Breaking Speed–And it’s Reusable
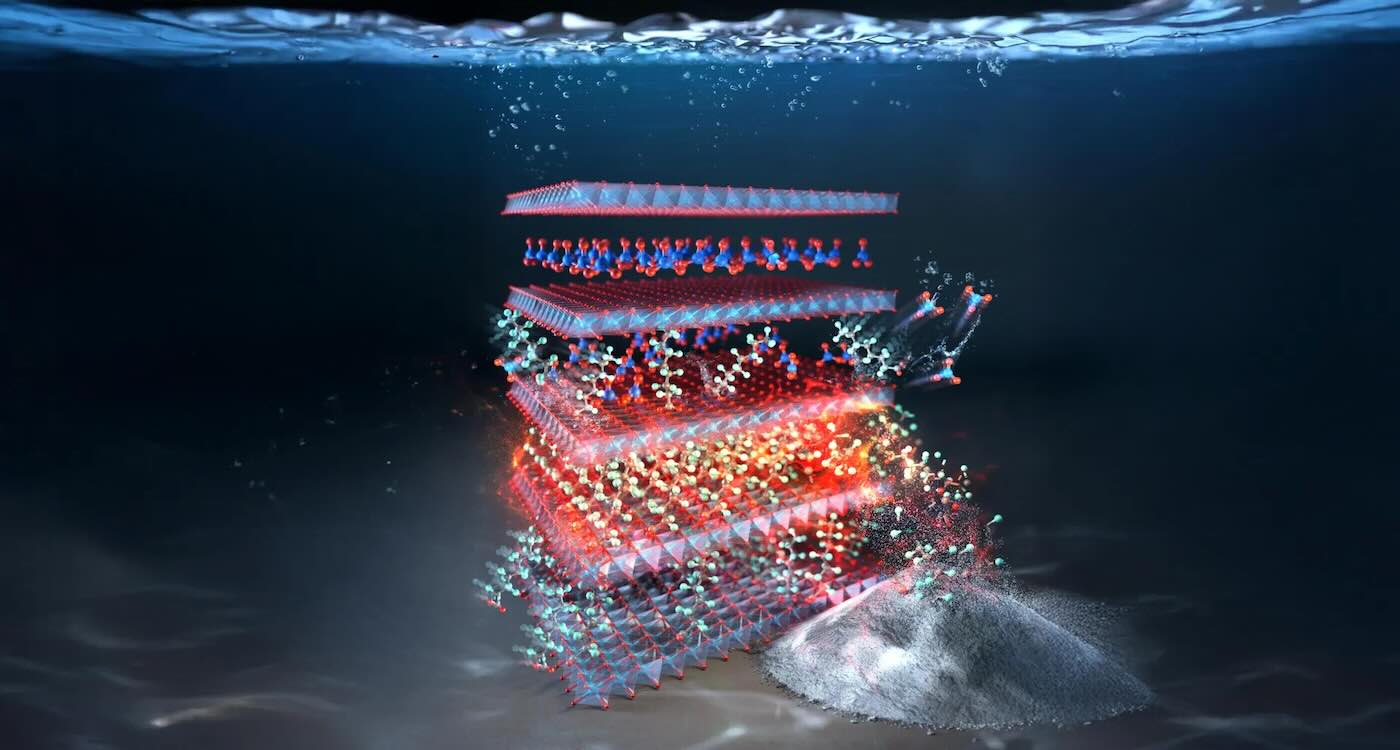 PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice University
PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice UniversityTime of day may determine heart surgery outcomes: Study

Mom and Baby Beat 1-in-a-Million Odds to Survive the ‘Rarest of Pregnancies’

A Rare Cancer-Fighting Plant Compound has Finally Been Decoded
 Anti-cancer plant enzyme uncovered by Tuan-Anh Nguyen and Dr Thu-Thuy Dang – UBC Okanagan
Anti-cancer plant enzyme uncovered by Tuan-Anh Nguyen and Dr Thu-Thuy Dang – UBC Okanagan Red vein kratom leaves by Jade at Thehealingeast – CC BY-SA 4.0
Red vein kratom leaves by Jade at Thehealingeast – CC BY-SA 4.0Simply Shining Light on Skin Can Replace Finger Pricks for People With Diabetes
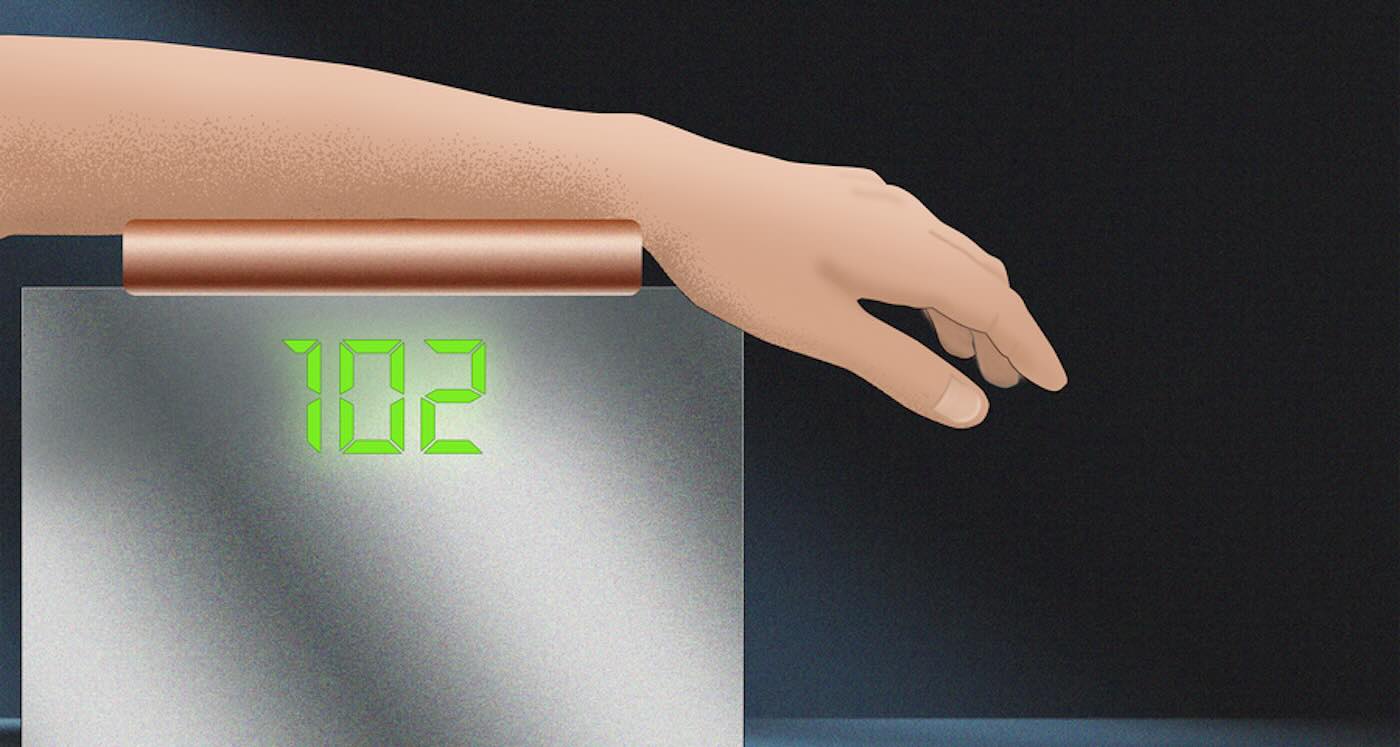 Blood-glucose monitor uses light to spare diabetes patients from finger pricks – Credit: Christine Daniloff / MIT
Blood-glucose monitor uses light to spare diabetes patients from finger pricks – Credit: Christine Daniloff / MITA Rare Cancer-Fighting Plant Compound has Finally Been Decoded


Patients Thought Untreatable with Rare Disease Dramatically Improve with Common Gene Therapy
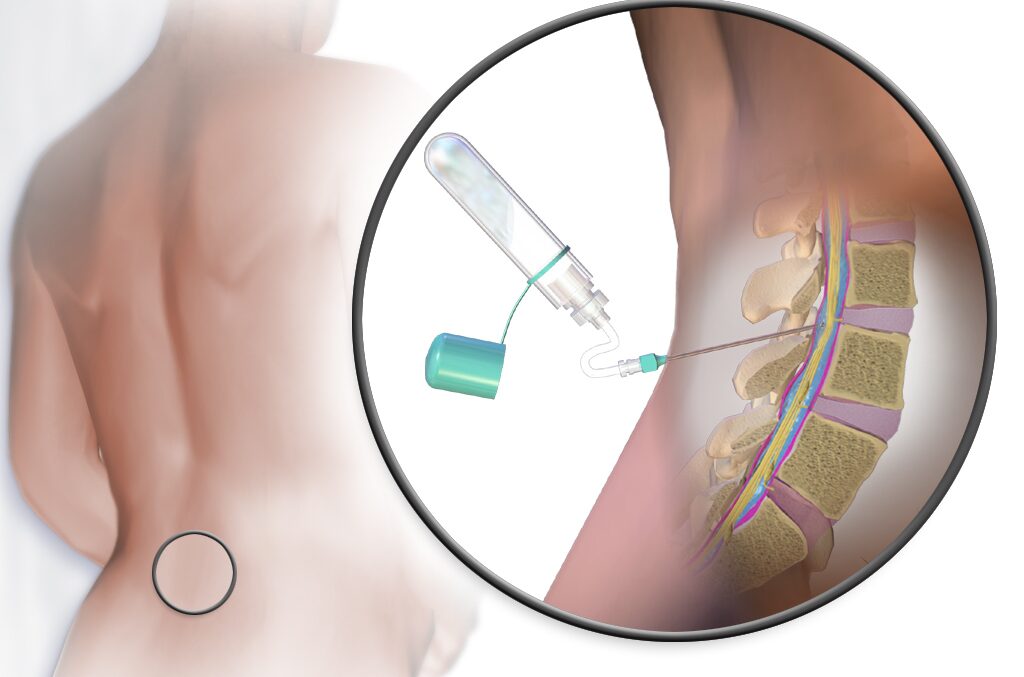
First Human Cornea Transplant Using 3D Printed, Lab-Grown Tissue Restores Sight in a ‘Game Changer’ for Millions Who are Blind
The first successful human implant of a 3D-printed cornea made from human eye cells cultured in a laboratory has restored a patient’s sight.
The North Carolina-based company that developed the cornea described the procedure as a ‘world first’—and a major milestone toward its goal of alleviating the lack of available donor tissue and long wait-times for people seeking transplants.
According to Precise Bio, its robotic bio-fabrication approach could potentially turn a single donated cornea into hundreds of lab-grown grafts, at a time when there’s currently only one available for an estimated 70 patients who need one to see.
“This achievement marks a turning point for regenerative ophthalmology—a moment of real hope for millions living with corneal blindness,” Aryeh Batt, Precise Bio’s co-founder and CEO, said in a statement.
“For the first time, a corneal implant manufactured entirely in the lab from cultured human corneal cells, rather than direct donor tissue, has been successfully implanted in a patient.”
The company said the transplant was performed Oct. 29 in one eye of a patient who was considered legally blind.
“This is a game changer. We’ve witnessed a cornea created in the lab, from living human cells, bring sight back to a human being,” said Dr. Michael Mimouni, director of the cornea unit at Rambam Medical Center in Israel, who performed the procedure.
“It was an unforgettable moment—a glimpse into a future where no one will have to live in darkness because of a shortage of donor tissue.”
Dubbed PB-001, the implant is designed to match the optical clarity, transparency and bio-mechanical properties of a native cornea. Previously tested in animal models, the company said its graft is capable of integrating with a patient’s own tissue.
The outer layer of the eye—covering the iris and pupil—can end up clouding a person’s vision following injuries, infections, scarring and other conditions. PB-001 is currently being tested in a single-arm phase 1 trial in Israel, which aims to enroll between 10 and 15 participants with excess fluid buildups in the cornea due to dysfunction within its inner cell layers.
Precise Bio said it plans to announce top-line results from the study in the second half of 2026, tracking six-month efficacy outcomes.
The corneas are designed to be compatible with current surgery hardware and workflows. Shipped under long-term cryopreservation, it is delivered preloaded on standard delivery devices and unrolls during implantation to form a natural corneal shape.
“PB-001 has the potential to offer a new, standardized solution to one of ophthalmology’s most urgent needs—reliable, safe, and effective corneal replacement,” said Anthony Atala, M.D., co-founder of Precise Bio and director of the Wake Forest Institute for Regenerative Medicine.
“The ability to produce patient-ready tissue on demand could lead the way towards reshaping transplant medicine as we know it.”(Edited from original article by Conor Hale) First Human Cornea Transplant Using 3D Printed, Lab-Grown Tissue Restores Sight in a ‘Game Changer’ for Millions Who are Blind
Indian study finds 1st evidence on how nanoplastics from single-use PET bottles harm body

COVID-19 mRNA vaccines could unlock the next revolution in cancer treatment – new research
The COVID-19 mRNA-based vaccines that saved 2.5 million lives globally during the pandemic could help spark the immune system to fight cancer. This is the surprising takeaway of a new study that we and our colleagues published in the journal Nature.
While developing mRNA vaccines for patients with brain tumors in 2016, our team, led by pediatric oncologist Elias Sayour, discovered that mRNA can train immune systems to kill tumors – even if the mRNA is not related to cancer.
Based on this finding, we hypothesized that mRNA vaccines designed to target the SARS-CoV-2 virus that causes COVID-19 might also have antitumor effects.
So we looked at clinical outcomes for more than 1,000 late-stage melanoma and lung cancer patients treated with a type of immunotherapy called immune checkpoint inhibitors. This treatment is a common approach doctors use to train the immune system to kill cancer. It does this by blocking a protein that tumor cells make to turn off immune cells, enabling the immune system to continue killing cancer.
Remarkably, patients who received either the Pfizer or Moderna mRNA-based COVID-19 vaccine within 100 days of starting immunotherapy were more than twice as likely to be alive after three years compared with those who didn’t receive either vaccine. Surprisingly, patients with tumors that don’t typically respond well to immunotherapy also saw very strong benefits, with nearly fivefold improvement in three-year overall survival. This link between improved survival and receiving a COVID-19 mRNA vaccine remained strong even after we controlled for factors like disease severity and co-occurring conditions.
To understand the underlying mechanism, we turned to animal models. We found that COVID-19 mRNA vaccines act like an alarm, triggering the body’s immune system to recognize and kill tumor cells and overcome the cancer’s ability to turn off immune cells. When combined, vaccines and immune checkpoint inhibitors coordinate to unleash the full power of the immune system to kill cancer cells.
Why it matters
Immunotherapy with immune checkpoint inhibitors has revolutionized cancer treatment over the past decade by producing cures in many patients who were previously considered incurable. However, these therapies are ineffective in patients with “cold” tumors that successfully evade immune detection.
Our findings suggest that mRNA vaccines may provide just the spark the immune system needs to turn these “cold” tumors “hot.” If validated in our upcoming clinical trial, our hope is that this widely available, low-cost intervention could extend the benefits of immunotherapy to millions of patients who otherwise would not benefit from this therapy.
Unlike vaccines for infectious diseases, which are used to prevent an infection, therapeutic cancer vaccines are used to help train the immune systems of cancer patients to better fight tumors.
We and many others are currently working hard to make personalized mRNA vaccines for patients with cancer. This involves taking a small sample of a patient’s tumor and using machine learning algorithms to predict which proteins in the tumor would be the best targets for a vaccine. However, this approach can be costly and difficult to manufacture.
In contrast, COVID-19 mRNA vaccines do not need to be personalized, are already widely available at low or no cost around the globe, and could be administered at any time during a patient’s treatment. Our findings that COVID-19 mRNA vaccines have substantial antitumor effects bring hope that they could help extend the anti-cancer benefits of mRNA vaccines to all.
What’s next
In pursuit of this goal, we are preparing to test this treatment strategy in patients with a nationwide clinical trial in people with lung cancer. People receiving an immune checkpoint inhibitor will be randomized to either receive a COVID-19 mRNA vaccine during treatment or not.
This study will tell us whether COVID-19 mRNA vaccines should be included as part of the standard of care for patients receiving an immune checkpoint inhibitor. Ultimately, we hope that this approach will help many patients who are treated with immune therapy, and especially those who currently lack effective treatment options.
This work exemplifies how a tool born from a global pandemic may provide a new weapon against cancer and rapidly extend the benefits of existing treatments to millions of patients. By harnessing a familiar vaccine in a new way, we hope to extend the lifesaving benefits of immunotherapy to cancer patients who were previously left behind.
The Research Brief is a short take on interesting academic work.![]()
Adam Grippin, Physician Scientist in Cancer Immunotherapy, The University of Texas MD Anderson Cancer Center and Christiano Marconi, Ph.D. Candidate in Immunotherapy, University of Florida
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Scorpion Venom May Provide the Next Breast Cancer Breakthrough
 – credit Marino Linic
– credit Marino LinicScientists in Brazil are currently testing to see if the venom of an Amazonian scorpion could be used to poison breast cancer tumors.
Researchers at the University of São Paulo’s Preto School of Pharmaceutical Sciences (FCFRP-USP) have long worked to clone and express proteins from rattlesnake and scorpion venom with hopes of transforming these powerful compounds into medicines.
Recently, their work identified that venom of the scorpion Brotheas amazonicus appears to attack breast cancer cells in a way similar to a widely used chemotherapy medication.
These early findings were generated through a collaboration with scientists from the National Institute for Amazonian Research (INPA) and the Amazonas State University (UEA).
“Through bioprospecting, we were able to identify a molecule in the species of this Amazonian scorpion that is similar to that found in the venoms of other scorpions and that acts against breast cancer cells,” said Eliane Candiani Arantes, a professor at FCFRP-USP and the coordinator of the project.
Arantes and her team identified two neurotoxins in scorpion venom with immunosuppressive effects. Working with collaborators at INPA and UEA, they found a peptide named BamazScplp1 in the venom of Brotheas amazonicus that appears to have anti-tumor potential.
Laboratory tests showed that the peptide’s impact on breast cancer cells was comparable to paclitaxel, a commonly prescribed chemotherapy treatment. It primarily triggers necrosis, a form of cell death previously associated with molecules from other scorpion species.
Arantes and her team have isolated other components of venoms from scorpions and from snakes that have been used to help develop other clinical applications, including an internal wound sealant that mimics the body’s natural clotting and scaffolding processes. It’s undergoing trials for use in nerve repair, bone healing, and restoring movement following spinal cord injury.Next time you see a scorpion, and think it a nasty creepy crawly that will send you to the hospital, show a bit of grace; they might help save a woman’s life some day. Scorpion Venom May Provide the Next Breast Cancer Breakthrough
Indian scientists find genetic clues to tackle oral cancer among women

Australia leads first human trial of one-time gene editing therapy to halve bad cholesterol

Boy with Rare Genetic Disorder Amazes Doctors After World-First Gene Therapy
 Courtesy of Oliver Chu family
Courtesy of Oliver Chu familyParkinson's disease causes progressive changes in brain's blood vessels: Study

Japanese researchers successfully regenerate bone using stem cells

Unique Antibody from Camels and Alpacas Could Be Used to Treat Alzheimer’s
 – credit, Sung Jin Cho on Unsplash
– credit, Sung Jin Cho on UnsplashNew online tool to transform how high blood pressure is treated
 IANS Photo
IANS PhotoNagaland University researchers find plant compound to treat diabetic wound, foot ulcers

