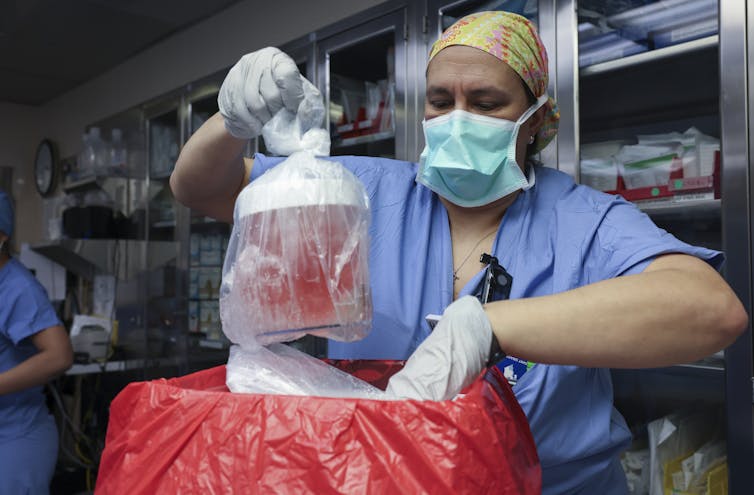
In a world first, we heard last week that US surgeons had transplanted a kidney from a gene-edited pig into a living human. News reports said the procedure was a breakthrough in xenotransplantation – when an organ, cells or tissues are transplanted from one species to another.
Champions of xenotransplantation regard it as the solution to organ shortages across the world. In December 2023, 1,445 people in Australia were on the waiting list for donor kidneys. In the United States, more than 89,000 are waiting for kidneys.
One biotech CEO says gene-edited pigs promise “an unlimited supply of transplantable organs”.
Not, everyone, though, is convinced transplanting animal organs into humans is really the answer to organ shortages, or even if it’s right to use organs from other animals this way.
There are two critical barriers to the procedure’s success: organ rejection and the transmission of animal viruses to recipients.
But in the past decade, a new platform and technique known as CRISPR/Cas9 – often shortened to CRISPR – has promised to mitigate these issues.
What is CRISPR?
CRISPR gene editing takes advantage of a system already found in nature. CRISPR’s “genetic scissors” evolved in bacteria and other microbes to help them fend off viruses. Their cellular machinery allows them to integrate and ultimately destroy viral DNA by cutting it.
In 2012, two teams of scientists discovered how to harness this bacterial immune system. This is made up of repeating arrays of DNA and associated proteins, known as “Cas” (CRISPR-associated) proteins.
When they used a particular Cas protein (Cas9) with a “guide RNA” made up of a singular molecule, they found they could program the CRISPR/Cas9 complex to break and repair DNA at precise locations as they desired. The system could even “knock in” new genes at the repair site.
In 2020, the two scientists leading these teams were awarded a Nobel prize for their work.
In the case of the latest xenotransplantation, CRISPR technology was used to edit 69 genes in the donor pig to inactivate viral genes, “humanise” the pig with human genes, and knock out harmful pig genes.
A busy time for gene-edited xenotransplantation
While CRISPR editing has brought new hope to the possibility of xenotransplantation, even recent trials show great caution is still warranted.
In 2022 and 2023, two patients with terminal heart diseases, who were ineligible for traditional heart transplants, were granted regulatory permission to receive a gene-edited pig heart. These pig hearts had ten genome edits to make them more suitable for transplanting into humans. However, both patients died within several weeks of the procedures.
Earlier this month, we heard a team of surgeons in China transplanted a gene-edited pig liver into a clinically dead man (with family consent). The liver functioned well up until the ten-day limit of the trial.
How is this latest example different?
The gene-edited pig kidney was transplanted into a relatively young, living, legally competent and consenting adult.
The total number of gene edits edits made to the donor pig is very high. The researchers report making 69 edits to inactivate viral genes, “humanise” the pig with human genes, and to knockout harmful pig genes.
Clearly, the race to transform these organs into viable products for transplantation is ramping up.
From biotech dream to clinical reality
Only a few months ago, CRISPR gene editing made its debut in mainstream medicine.
In November, drug regulators in the United Kingdom and US approved the world’s first CRISPR-based genome-editing therapy for human use – a treatment for life-threatening forms of sickle-cell disease.
The treatment, known as Casgevy, uses CRISPR/Cas-9 to edit the patient’s own blood (bone-marrow) stem cells. By disrupting the unhealthy gene that gives red blood cells their “sickle” shape, the aim is to produce red blood cells with a healthy spherical shape.
Although the treatment uses the patient’s own cells, the same underlying principle applies to recent clinical xenotransplants: unsuitable cellular materials may be edited to make them therapeutically beneficial in the patient.
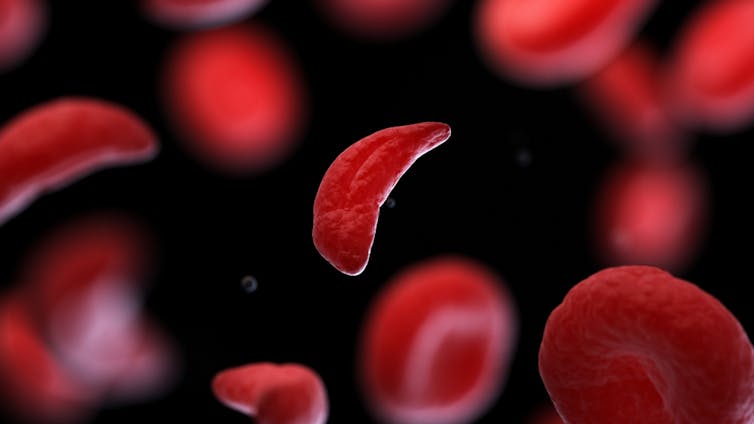 CRISPR technology is aiming to restore diseased red blood cells to their healthy round shape. Sebastian Kaulitzki/Shutterstock
CRISPR technology is aiming to restore diseased red blood cells to their healthy round shape. Sebastian Kaulitzki/ShutterstockWe’ll be talking more about gene-editing
Medicine and gene technology regulators are increasingly asked to approve new experimental trials using gene editing and CRISPR.
However, neither xenotransplantation nor the therapeutic applications of this technology lead to changes to the genome that can be inherited.
For this to occur, CRISPR edits would need to be applied to the cells at the earliest stages of their life, such as to early-stage embryonic cells in vitro (in the lab).
In Australia, intentionally creating heritable alterations to the human genome is a criminal offence carrying 15 years’ imprisonment.
No jurisdiction in the world has laws that expressly permits heritable human genome editing. However, some countries lack specific regulations about the procedure.
Is this the future?
Even without creating inheritable gene changes, however, xenotransplantation using CRISPR is in its infancy.
For all the promise of the headlines, there is not yet one example of a stable xenotransplantation in a living human lasting beyond seven months.
While authorisation for this recent US transplant has been granted under the so-called “compassionate use” exemption, conventional clinical trials of pig-human xenotransplantation have yet to commence.
But the prospect of such trials would likely require significant improvements in current outcomes to gain regulatory approval in the US or elsewhere.
By the same token, regulatory approval of any “off-the-shelf” xenotransplantation organs, including gene-edited kidneys, would seem some way off.![]()
Christopher Rudge, Law lecturer, University of Sydney
This article is republished from The Conversation under a Creative Commons license. Read the original article.