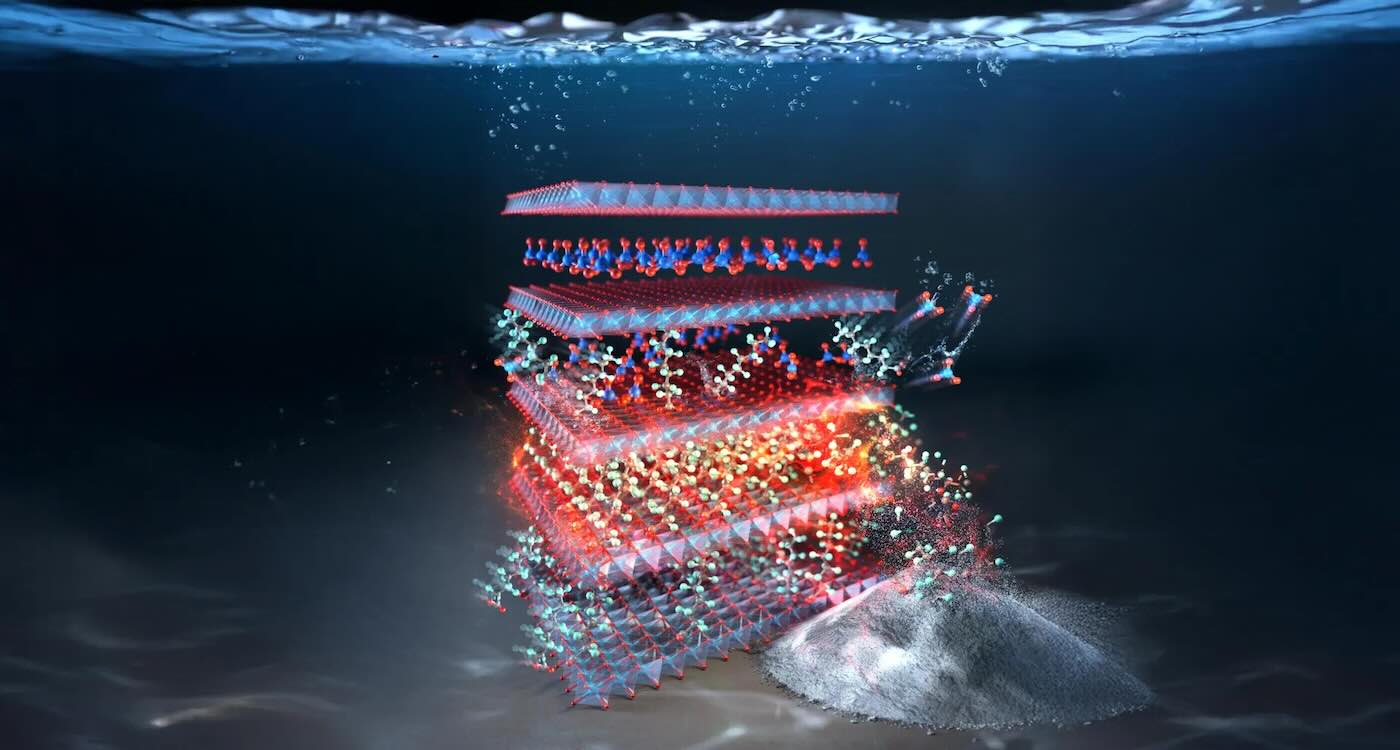
 PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice University
PFAs self-destruct in this layered double hydroxide material made from copper and aluminum – credit: Rice UniversityUniversity researchers in Texas and Korea have collaborated to developed an eco-friendly water purifier that captures—and destroys—toxic “forever chemicals” (PFAS) more than 1,000 times better than current methods.
Their study marks a major milestone in addressing one of the world’s most persistent environmental and health threats.
PFAS are synthetic chemicals first created in the 1940s for use in products ranging from Teflon pans to waterproof clothing and food packaging. Their ability to resist heat, grease, and water has made them valuable for industry and consumers, but that same resistance means they do not easily degrade.
Current health studies have suggested their lingering residues in water are linked to possible liver damage, reproductive disorders, immune system disruption, and certain cancers.
Traditional PFAS cleanup methods typically rely on adsorption, where molecules cling to materials like activated carbon or ion-exchange resins. While these methods are widely used, they come with major drawbacks: low efficiency, slow performance, and the creation of additional waste that requires disposal.
“Our new approach offers a sustainable and highly effective alternative,” said Professor Michael Wong at Rice University, who specializes in nanotechnology, chemistry, and biomolecular engineering.
The innovation centers on a layered double hydroxide (LDH) material made from copper and aluminum, first discovered by South Korean Professor Keon-Ham Kim, while he was a grad student at Korea Advanced Institute of Science and Technology in 2021.
While experimenting with these materials, a student at Rice, Youngkun Chung, discovered that one formulation with nitrate could adsorb PFAS with record-breaking efficiency.
“To my astonishment, this LDH compound captured PFAS more than 1,000 times better than other materials,” said Chung, a lead author of the study.
“It also worked incredibly fast, removing large amounts of PFAS within minutes, about 100 times faster than commercial carbon filters.”
The material’s effectiveness stems from its unique internal structure.
Its organized copper-aluminum layers combined with slight charge imbalances create an ideal environment for PFAS molecules to bind—with both speed and strength.
Works equally well in river water, tap water and wastewater
To test the technology’s practicality, the team evaluated the LDH material in river water, tap water and wastewater. In all cases, it proved highly effective, performing well in both static and continuous-flow systems.
The results, recently published in the journal Advanced Materials, suggest strong potential for large-scale applications in municipal water treatment and industrial cleanup.
Closing the waste loop
Removing PFAS from water is only part of the challenge. Destroying them safely is equally important. The team at Rice developed a method to thermally decompose PFAS captured on the LDH material. By heating the saturated material with calcium carbonate, the team eliminated more than half of the trapped PFAS without releasing toxic by-products.
Remarkably, the process also regenerated the LDH, allowing it to be reused multiple times—refreshing itself for reuse.
“It’s a rare one-two punch against pollution,” wrote Science Daily, “fast cleanup and sustainable destruction.”
Preliminary studies showed the material could complete at least six full cycles of capture, destruction and renewal, making it the first known eco-friendly, sustainable system for PFAS removal.
“We are excited by the potential of this one-of-a-kind LDH-based technology to transform how PFAS-contaminated water sources are treated in the near future,” said Professor Wong said.“It’s the result of an extraordinary international collaboration and the creativity of young researchers.” New Eco-Friendly Tech Eliminates ‘Forever Chemicals’ With Record-Breaking Speed–And it’s Reusable

