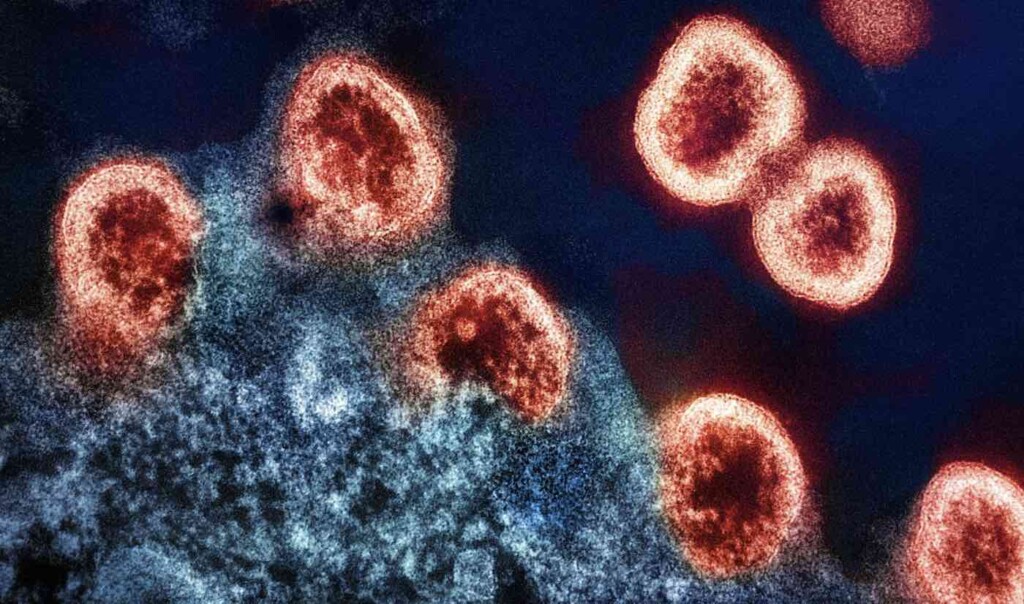
 HIV-1 virus particles under electron micrograph with H9 T-cells (in blue) – Credit: National Institute of Allergy and Infectious Diseases
HIV-1 virus particles under electron micrograph with H9 T-cells (in blue) – Credit: National Institute of Allergy and Infectious Diseases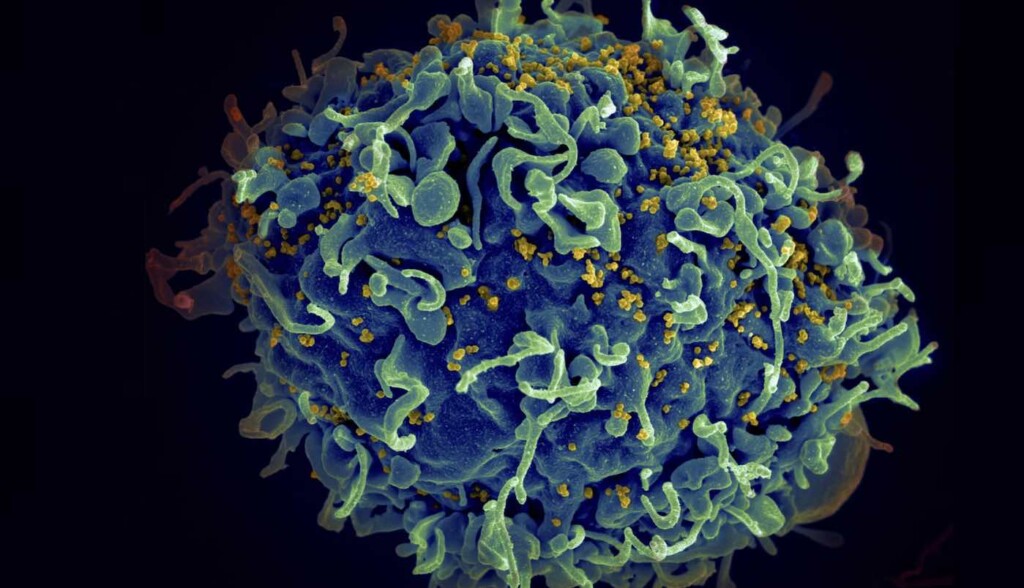
 HIV-1 virus particles under electron micrograph with H9 T-cells (in blue) – Credit: National Institute of Allergy and Infectious Diseases
HIV-1 virus particles under electron micrograph with H9 T-cells (in blue) – Credit: National Institute of Allergy and Infectious Diseases
 Zebra finches are highly social birds and will press a lever in order to hear a recording of another Zebra finch singing. (Photo by Carlos Rodríguez-Saltos)
Zebra finches are highly social birds and will press a lever in order to hear a recording of another Zebra finch singing. (Photo by Carlos Rodríguez-Saltos)Since the first detonation of an atomic bomb in 1945, more than 2,000 nuclear weapons tests have been conducted by eight countries: the United States, the Soviet Union, the United Kingdom, France, China, India, Pakistan and North Korea.
Groups such as the Comprehensive Nuclear-Test-Ban Treaty Organization are constantly on the lookout for new tests. However, for reasons of safety and secrecy, modern nuclear tests are carried out underground – which makes them difficult to detect. Often, the only indication they have occurred is from the seismic waves they generate.
In a paper published in Geophysical Journal International, my colleagues and I have developed a way to distinguish between underground nuclear tests and natural earthquakes with around 99% accuracy.
The invention of nuclear weapons sparked an international arms race, as the Soviet Union, the UK and France developed and tested increasingly larger and more sophisticated devices in an attempt to keep up with the US.
Many early tests caused serious environmental and societal damage. For example, the US’s 1954 Castle Bravo test, conducted in secret at Bikini Atoll in the Marshall Islands, delivered large volumes of radioactive fallout to several nearby islands and their inhabitants.
Between 1952 and 1957, the UK conducted several tests in Australia, scattering long-lived radioactive material over wide areas of South Australian bushland, with devastating consequences for local Indigenous communities.
In 1963, the US, the UK and the USSR agreed to carry out future tests underground to limit fallout. Nevertheless, testing continued unabated as China, India, Pakistan and North Korea also entered the fray over the following decades.
During this period there were substantial international efforts to figure out how to monitor nuclear testing. The competitive nature of weapons development means much research and testing is conducted in secret.
Groups such as the Comprehensive Nuclear-Test-Ban Treaty Organization today run global networks of instruments specifically designed to identify any potential tests. These include:
Seismometers are designed to measure seismic waves: tiny vibrations of the ground surface generated when large amounts of energy are suddenly released underground, such as during earthquakes or nuclear explosions.
There are two main kinds of seismic waves. First are body waves, which travel outwards in all directions, including down into the deep Earth, before returning to the surface. Second are surface waves, which travel along Earth’s surface like ripples spreading out on a pond.
The difficulty in using seismic waves to monitor underground nuclear tests is distinguishing between explosions and naturally occurring earthquakes. A core goal of monitoring is never to miss an explosion, but there are thousands of sizeable natural quakes around the world every day.
As a result, monitoring underground tests is like searching for a potentially non-existent needle in a haystack the size of a planet.
Many different methods have been developed to aid this search over the past 60 years.
Some of the simplest include analysing the location or depth of the source. If an event occurs far from volcanoes and plate tectonic boundaries, it might be considered more suspicious. Alternatively, if it occurs at a depth greater than say three kilometres, it is unlikely to have been a nuclear test.
However, these simple methods are not foolproof. Tests might be carried out in earthquake-prone areas for camouflage, for example, and shallow earthquakes are also possible.
A more sophisticated monitoring approach involves calculating the ratio of the amount of the energy transmitted in body waves to the amount carried in surface waves. Earthquakes tend to expend more of their energy in surface waves than explosions do.
This method has proven highly effective for identifying underground nuclear tests, but it too is imperfect. It failed to effectively classify the 2017 North Korean nuclear test, which generated substantial surface waves because it was carried out inside a tunnel in a mountain.
This outcome underlines the importance of using multiple independent discrimination techniques during monitoring – no single method is likely to prove reliable for all events.
In 2023, my colleagues and I from the Australian National University and Los Alamos National Laboratory in the US got together to re-examine the problem of determining the source of seismic waves.
We used a recently developed approach to represent how rocks are displaced at the source of a seismic event, and combined it with a more advanced statistical model to describe different types of event. As a result, we were able to take advantage of fundamental differences between the sources of explosions and earthquakes to develop an improved method of classifying these events.
We tested our approach on catalogues of known explosions and earthquakes from the western United States, and found that the method gets it right around 99% of the time. This makes it a useful new tool in efforts to monitor underground nuclear tests.
Robust techniques for identification of nuclear tests will continue to be a key component of global monitoring programs. They are critical for ensuring governments are held accountable for the environmental and societal impacts of nuclear weapons testing.![]()
Mark Hoggard, DECRA Research Fellow, Australian National University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
 Shutterstock
Bryan G. Fry, The University of Queensland
Shutterstock
Bryan G. Fry, The University of QueenslandThe green anaconda has long been considered one of the Amazon’s most formidable and mysterious animals. Our new research upends scientific understanding of this magnificent creature, revealing it is actually two genetically different species. The surprising finding opens a new chapter in conservation of this top jungle predator.
Green anacondas are the world’s heaviest snakes, and among the longest. Predominantly found in rivers and wetlands in South America, they are renowned for their lightning speed and ability to asphyxiate huge prey then swallow them whole.
My colleagues and I were shocked to discover significant genetic differences between the two anaconda species. Given the reptile is such a large vertebrate, it’s remarkable this difference has slipped under the radar until now.
Conservation strategies for green anacondas must now be reassessed, to help each unique species cope with threats such as climate change, habitat degradation and pollution. The findings also show the urgent need to better understand the diversity of Earth’s animal and plant species before it’s too late.
 Scientists discovered a new snake species known as the northern green anaconda. Bryan Fry
Scientists discovered a new snake species known as the northern green anaconda. Bryan FryHistorically, four anaconda species have been recognised, including green anacondas (also known as giant anacondas).
Green anacondas are true behemoths of the reptile world. The largest females can grow to more than seven metres long and weigh more than 250 kilograms.
The snakes are well-adapted to a life lived mostly in water. Their nostrils and eyes are on top of their head, so they can see and breathe while the rest of their body is submerged. Anacondas are olive-coloured with large black spots, enabling them to blend in with their surroundings.
The snakes inhabit the lush, intricate waterways of South America’s Amazon and Orinoco basins. They are known for their stealth, patience and surprising agility. The buoyancy of the water supports the animal’s substantial bulk and enables it to move easily and leap out to ambush prey as large as capybaras (giant rodents), caimans (reptiles from the alligator family) and deer.
Green anacondas are not venomous. Instead they take down prey using their large, flexible jaws then crush it with their strong bodies, before swallowing it.
As apex predators, green anacondas are vital to maintaining balance in their ecosystems. This role extends beyond their hunting. Their very presence alters the behaviour of a wide range of other species, influencing where and how they forage, breed and migrate.
Anacondas are highly sensitive to environmental change. Healthy anaconda populations indicate healthy, vibrant ecosystems, with ample food resources and clean water. Declining anaconda numbers may be harbingers of environmental distress. So knowing which anaconda species exist, and monitoring their numbers, is crucial.
To date, there has been little research into genetic differences between anaconda species. Our research aimed to close that knowledge gap.
 Green anaconda have large, flexible jaws. Pictured: a green anaconda eating a deer. JESUS RIVAS
Green anaconda have large, flexible jaws. Pictured: a green anaconda eating a deer. JESUS RIVASWe studied representative samples from all anaconda species throughout their distribution, across nine countries.
Our project spanned almost 20 years. Crucial pieces of the puzzle came from samples we collected on a 2022 expedition to the Bameno region of Baihuaeri Waorani Territory in the Ecuadorian Amazon. We took this trip at the invitation of, and in collaboration with, Waorani leader Penti Baihua. Actor Will Smith also joined the expedition, as part of a series he is filming for National Geographic.
We surveyed anacondas from various locations throughout their ranges in South America. Conditions were difficult. We paddled up muddy rivers and slogged through swamps. The heat was relentless and swarms of insects were omnipresent.
We collected data such as habitat type and location, and rainfall patterns. We also collected tissue and/or blood from each specimen and analysed them back in the lab. This revealed the green anaconda, formerly believed to be a single species, is actually two genetically distinct species.
The first is the known species, Eunectes murinus, which lives in Perú, Bolivia, French Guiana and Brazil. We have given it the common name “southern green anaconda”. The second, newly identified species is Eunectes akayima or “northern green anaconda”, which is found in Ecuador, Colombia, Venezuela, Trinidad, Guyana, Suriname and French Guiana.
We also identified the period in time where the green anaconda diverged into two species: almost 10 million years ago.
The two species of green anaconda look almost identical, and no obvious geographical barrier exists to separate them. But their level of genetic divergence – 5.5% – is staggering. By comparison, the genetic difference between humans and apes is about 2%.
 The two green anaconda species live much of their lives in water. Shutterstock
The two green anaconda species live much of their lives in water. ShutterstockOur research has peeled back a layer of the mystery surrounding green anacondas. This discovery has significant implications for the conservation of these species – particularly for the newly identified northern green anaconda.
Until now, the two species have been managed as a single entity. But each may have different ecological niches and ranges, and face different threats.
Tailored conservation strategies must be devised to safeguard the future of both species. This may include new legal protections and initiatives to protect habitat. It may also involve measures to mitigate the harm caused by climate change, deforestation and pollution — such as devastating effects of oil spills on aquatic habitats.
Our research is also a reminder of the complexities involved in biodiversity conservation. When species go unrecognised, they can slip through the cracks of conservation programs. By incorporating genetic taxonomy into conservation planning, we can better preserve Earth’s intricate web of life – both the species we know today, and those yet to be discovered.![]()
Bryan G. Fry, Professor of Toxicology, School of the Environment, The University of Queensland
This article is republished from The Conversation under a Creative Commons license. Read the original article.
 Arlington Research/Unsplash Peter Wilson, Australian Catholic UniversityWe’re all time-poor, so multi-tasking is seen as a necessity of modern living. We answer work emails while watching TV, make shopping lists in meetings and listen to podcasts when doing the dishes. We attempt to split our attention countless times a day when juggling both mundane and important tasks.
Arlington Research/Unsplash Peter Wilson, Australian Catholic UniversityWe’re all time-poor, so multi-tasking is seen as a necessity of modern living. We answer work emails while watching TV, make shopping lists in meetings and listen to podcasts when doing the dishes. We attempt to split our attention countless times a day when juggling both mundane and important tasks.
But doing two things at the same time isn’t always as productive or safe as focusing on one thing at a time.
The dilemma with multi-tasking is that when tasks become complex or energy-demanding, like driving a car while talking on the phone, our performance often drops on one or both.
Here’s why – and how our ability to multi-task changes as we age.
The issue with multi-tasking at a brain level, is that two tasks performed at the same time often compete for common neural pathways – like two intersecting streams of traffic on a road.
In particular, the brain’s planning centres in the frontal cortex (and connections to parieto-cerebellar system, among others) are needed for both motor and cognitive tasks. The more tasks rely on the same sensory system, like vision, the greater the interference.
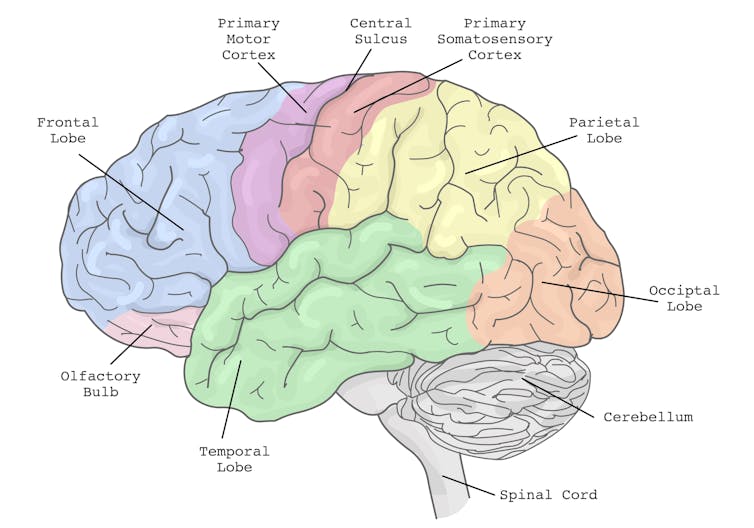 The brain’s action planning centres are in the frontal cortex (blue), with reciprocal connections to parietal cortex (yellow) and the cerebellum (grey), among others. grayjay/Shutterstock
The brain’s action planning centres are in the frontal cortex (blue), with reciprocal connections to parietal cortex (yellow) and the cerebellum (grey), among others. grayjay/ShutterstockThis is why multi-tasking, such as talking on the phone, while driving can be risky. It takes longer to react to critical events, such as a car braking suddenly, and you have a higher risk of missing critical signals, such as a red light.
The more involved the phone conversation, the higher the accident risk, even when talking “hands-free”.
 Having a conversation while driving slows your reaction time. GBJSTOCK/Shutterstock
Having a conversation while driving slows your reaction time. GBJSTOCK/ShutterstockGenerally, the more skilled you are on a primary motor task, the better able you are to juggle another task at the same time. Skilled surgeons, for example, can multitask more effectively than residents, which is reassuring in a busy operating suite.
Highly automated skills and efficient brain processes mean greater flexibility when multi-tasking.
Both brain capacity and experience endow adults with a greater capacity for multi-tasking compared with children.
You may have noticed that when you start thinking about a problem, you walk more slowly, and sometimes to a standstill if deep in thought. The ability to walk and think at the same time gets better over childhood and adolescence, as do other types of multi-tasking.
When children do these two things at once, their walking speed and smoothness both wane, particularly when also doing a memory task (like recalling a sequence of numbers), verbal fluency task (like naming animals) or a fine-motor task (like buttoning up a shirt). Alternately, outside the lab, the cognitive task might fall by wayside as the motor goal takes precedence.
Brain maturation has a lot to do with these age differences. A larger prefrontal cortex helps share cognitive resources between tasks, thereby reducing the costs. This means better capacity to maintain performance at or near single-task levels.
The white matter tract that connects our two hemispheres (the corpus callosum) also takes a long time to fully mature, placing limits on how well children can walk around and do manual tasks (like texting on a phone) together.
For a child or adult with motor skill difficulties, or developmental coordination disorder, multi-tastking errors are more common. Simply standing still while solving a visual task (like judging which of two lines is longer) is hard. When walking, it takes much longer to complete a path if it also involves cognitive effort along the way. So you can imagine how difficult walking to school could be.
Older adults are more prone to multi-tasking errors. When walking, for example, adding another task generally means older adults walk much slower and with less fluid movement than younger adults.
These age differences are even more pronounced when obstacles must be avoided or the path is winding or uneven.
 Our ability to multi-task reduces with age. Shutterstock/Grizanda
Our ability to multi-task reduces with age. Shutterstock/GrizandaOlder adults tend to enlist more of their prefrontal cortex when walking and, especially, when multi-tasking. This creates more interference when the same brain networks are also enlisted to perform a cognitive task.
These age differences in performance of multi-tasking might be more “compensatory” than anything else, allowing older adults more time and safety when negotiating events around them.
Testing multi-tasking capabilities can tell clinicians about an older patient’s risk of future falls better than an assessment of walking alone, even for healthy people living in the community.
Testing can be as simple as asking someone to walk a path while either mentally subtracting by sevens, carrying a cup and saucer, or balancing a ball on a tray.
Patients can then practise and improve these abilities by, for example, pedalling an exercise bike or walking on a treadmill while composing a poem, making a shopping list, or playing a word game.
The goal is for patients to be able to divide their attention more efficiently across two tasks and to ignore distractions, improving speed and balance.
Let’s not forget that a good walk can help unclutter our mind and promote creative thought. And, some research shows walking can improve our ability to search and respond to visual events in the environment.
We often overlook the emotional and energy costs of multi-tasking when time-pressured. In many areas of life – home, work and school – we think it will save us time and energy. But the reality can be different.
Multi-tasking can sometimes sap our reserves and create stress, raising our cortisol levels, especially when we’re time-pressured. If such performance is sustained over long periods, it can leave you feeling fatigued or just plain empty.
Deep thinking is energy demanding by itself and so caution is sometimes warranted when acting at the same time – such as being immersed in deep thought while crossing a busy road, descending steep stairs, using power tools, or climbing a ladder.
So, pick a good time to ask someone a vexed question – perhaps not while they’re cutting vegetables with a sharp knife. Sometimes, it’s better to focus on one thing at a time.![]()
Peter Wilson, Professor of Developmental Psychology, Australian Catholic University
This article is republished from The Conversation under a Creative Commons license. Read the original article.